
206 High St, Suite 2
PO Box 726
New Windsor, MD 21776
410-775-7060 (phone)
410-775-7061 (fax)
 ImmTech has extensive experience in the development and manufacture of kits and reagents for use in biodetection assays, including an antigen detection kit for the detection of Influenza A nucleoprotein, and a variety of substrates, buffers, and accessory reagents that can be used to enhance the performance of enzyme immunoassays (photometric as well as chemiluminescent), immunofluorescent assays and immunohistochemical assays.
ImmTech has extensive experience in the development and manufacture of kits and reagents for use in biodetection assays, including an antigen detection kit for the detection of Influenza A nucleoprotein, and a variety of substrates, buffers, and accessory reagents that can be used to enhance the performance of enzyme immunoassays (photometric as well as chemiluminescent), immunofluorescent assays and immunohistochemical assays.
A variety of wild waterfowl appear to be the predominant natural reservoir for Influenza A viruses and subtypes representing many of the hemagglutinin and neuraminidase combinations can be found circulating in these birds. Historically, human influenza virus infections have been associated with H1N1, H2N2, and H3N2 subtypes of influenza A, although a recent (1997) and significant outbreak in Hong Kong was identified as an H5N1 subtype. This outbreak was not only significant because it resulted in 18 human infections and 6 deaths, but it also represented the first known demonstration of avian influenza virus transmission to humans. Since the 1997 H5N1 outbreak in Hong Kong, additional outbreaks of different influenza A subtypes exhibiting bird to human transmission have been documented; H9N2 in Hong Kong (1999, 2003) and China (1999), H7N2 in The Netherlands (2003), and H5N1 in China (2003) and Southeast Asia (2003-2004). As a result of these outbreaks and other information, there is a significant concern that a human pandemic strain of influenza A could emerge.
While Influenza A subtype identification is extremely important (vaccine production, epidemiology), the rapid and accurate differentiation of influenza A from influenza B and C and other repiratory agents in humans and animals is also important (treatment and biosecurity). ImmTech has developed a highly sensitive and specific enzyme immunoassay for the detection of Influenza A nucleoprotein antigen in complex sample matrices derived from both human and veterinary sources. The assay can be completed in less than 1.5 hr. and contains only one wash step.
The ImmTech Influenza A Nucleoprotein Antigen Detection EIA also incorporates proprietary diluents that are designed to prevent the development of nonspecific signal derived from complex sample matrix effects or the nonspecific adsorption of reactive test components resulting in both high sensitivity and specificity (Figure 1-5). The test kit is available in a standard photometric format that utilizes TMB (3,3',5,5'-tetramethylbenzidine), a highly sensitive, photometric horseradish peroxidase (HRP) substrate (Figure 1) or in an HRP-based chemiluminescent format for enhanced sensitivity (Figure 2). Influenza A subtype sensitivity is demonstrated in Figure 5. The efficacy of the proprietary ImmTech diluent can be seen in Figures 3 and 4, a variety of poultry samples (tracheal and cloacal swabs, tissue homogenates, litter samples, and allantoic samples) were tested using a standard diluent and the kit diluent. The results clearly show the benefit of the kit diluent. Likewise, a series of tracheal and cloacal swabs from noninfected chickens were tested (Figure 4) and the nonspecific activity associated with these types of samples was eliminated as well. Additional specificity testing was performed using high titers of the following potentially interfering or cross-reactive agents and no reactivity was detected:
- Infectious Bronchitis Virus (Beaudette)
- Infectious Bursal Disease Virus (2512)
- Infectious Laryngotracheitis Virus (Cover)
- Newcastle Disease Virus (LaSota)
- Influenza B
- Respiratory Syncytial Virus
- Parainfluenza Virus, Type 1
- Parainfluenza Virus, Type 2
- Parainfluenza Virus, Type 3
- E. coli
- S. typhimurium
- C. jejuni
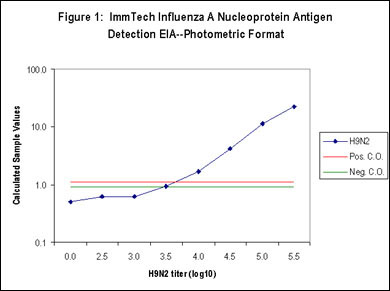
Figure 1: Photmetric format
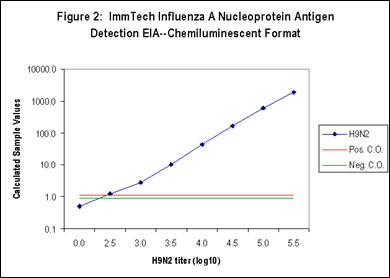
Figure 2: Chemiluminescent format
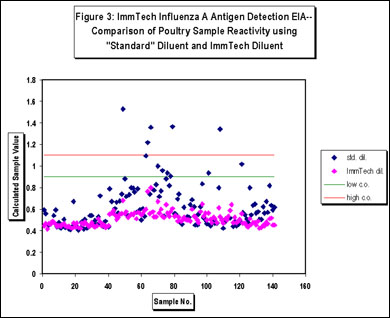
Figure 3: Photometric format
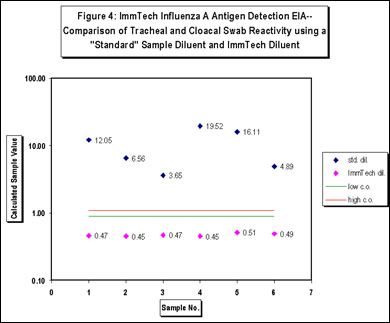
Figure 4: Photometric format
Antigen Detection EIA--Influenza A Subtype Detection
| Subtype | Designation | EIA Result |
| H1N1 | A/Sw/Iowa/31 | Reactive |
| H2N3 | A/Mal/Al/77 | Reactive |
| H2N9 | A/Pintail/Alb/293/77 | Reactive |
| H3N2 | A/Ty/Eng/69 | Reactive |
| H3N8 | A/Dk/Ukraine/1/63* | Reactive |
| H4N8 | A/Dk/Czech/56* | Reactive |
| H5N1 | A/Ck/Scot/59 | Reactive |
| H5N2 | A/Quail/Ore/20719/86 | Reactive |
| H5N2 | A/Ck/Wa/13413/84 | Reactive |
| H5N2 | A/Ck/Penn/13701/83 | Reactive |
| H5N2 | A/Ty/Min/3689-1551/81 | Reactive |
| H5N3 | A/Ty/cA/35621/84 | Reactive |
| H5N3 | A/Tern/SA/61* | Reactive |
| H5N8 | A/Ty/Ireland/83 | Reactive |
| H5N9 | A/Ty/Wis/68 | Reactive |
| H5N9 | A/Ty/Ont/7732/66 | Reactive |
| H5N9 | A/Ck/Que/14588-19 (Mex. Isolate) | Reactive |
| H5N2 | A/Ck/Hidalgo/26654-1368/94 (Mex. Isolate) | Reactive |
| H5N2 | A/Ck/Pue/8623-607 (Mex. Isolate) | Reactive |
| H5N? | A/Emu/Tx/39924/93 | Reactive |
| H5N3 | A/Emu/Tx/39924/93 (IB clone E2) | Reactive |
| H6N2 | Field Isolate, Cnn00053 | Reactive |
| H6N8 | A/Ty/Ont/63 | Reactive |
| H7N2 | A/Ty/Ore/71 | Reactive |
| H7N3 | A/Ck/Aust/3634/92 | Reactive |
| H7N3 | A/Ty/MN/29206/83 | Reactive |
| H7N7 | A/Ck/Vic/32972/85 | Reactive |
| H7N8 | A/Magrob/China/28710/93 | Reactive |
| H7N9 | A/Ty/MN/38429/88 | Reactive |
| H8N4 | A/Ty/Ont/61181/67* | Reactive |
| H9N2 | A/Ty/MN/12877/1285/81 | Reactive |
| H9N2 | A/Ty/Wis/1/66* | Reactive |
| H9N9 | A/Pheasent/Wa/37 | Reactive |
| H10N7 | A/Ck/Germany/49* | Reactive |
| H10N8 | A/Quail/Ithaca/1117/65 | Reactive |
| H11N1 | A/Dk/Eng/56* | Reactive |
| H11N9 | A/Dk/Memphis/546/74 | Reactive |
| H12N1 | A/Dk/Alberta/60/76* | Reactive |
| H13N1 | A/Gull/MD/704/77* | Reactive |
| H14N5 | A/Mal/Gurjev/263/83* | Reactive |
| Catalog No. | Size | Price (USD) |
| IAV-1192 | 2 plates (192 tests) | $495.00 |
| IAV-1480 | 5 plates (480 tests) | $1095.00 |
| IAV-2192 | 2 plates (192 tests) | $595.00 | IAV-2480 | 5 plates (480 tests) | $1295.00 |
The Influenza A Nucleoprotein Antigen Concentrate supplied in this product was produced using A/Beijing/262/95 (H1N1). The virus was grown in embryonated eggs, detergent-treated to remove the viral envelope and concentrated by centrifugation. Following SDS-PAGE, nucleoprotein concentration was calculated as a percentage of the total protein in the preparation.
| Catalog No. | Size | Price (USD) |
| NCP-1050 | 10 assays | $195.00 |
A variety of wild waterfowl appear to be the predominant natural reservoir for Influenza A viruses and subtypes representing most of the hemagglutinin and neuraminidase combinations can be found circulating in these birds. Historically, human influenza virus infections have been associated with H1N1, H2N2, and H3N2 subtypes of influenza A, although a recent (1997) and significant outbreak in Hong Kong was identified as an H5N1 subtype. This outbreak was not only significant because it resulted in 18 human infections and 6 deaths, but it also represented the first known demonstration of avian influenza virus transmission to humans.
Depending upon the serological requirements or research interest (natural infection, vaccine monitoring, or DIVA [Differentiation of Infected from Vaccinated Animals]), it may be useful to monitor the development of influenza A NP-specific antibody in a variety of species. ImmTech has developed a highly sensitive and specific enzyme-linked immunosorbent assay (ELISA) for the detection of influenza A NP-specific antibodies in serum from human and veterinary sources. Because the assay utilizes "inhibition of binding" technology, it may be used with serum from any species, and therefore does not require species-specific conjugates. The assay can be completed in less than 1.5 hr., contains only one wash step, and incorporates proprietary diluents that are designed to prevent the development of nonspecific signal derived from sample matrix and/or the nonspecific adsorption of reactive test components. The result is an assay that is both highly sensitive and specific.
| Catalog No. | Size | Price (USD) |
| IAB-1192 | 2 plates (192 tests) | $495.00 |
| IAB-1480 | 5 plates (480 tests) | $995.00 |
| Catalog No. | Size | Price (USD) |
| DIL-2001 | 25 ml | $45.00 |
| DIL-2001 | 100 ml | $150.00 |